Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3


Chemistry, 23.06.2019 04:31
What is the amount of energy for a photon that has a 125 cm wavelength
Answers: 2
You know the right answer?
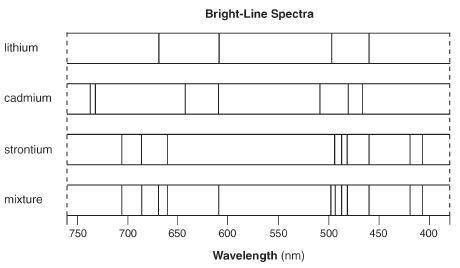
Answer the following question based on the bright line spectra. Explain, in terms of both electrons...
Questions




Mathematics, 26.03.2020 20:18




Social Studies, 26.03.2020 20:18





Social Studies, 26.03.2020 20:18


Biology, 26.03.2020 20:18





Mathematics, 26.03.2020 20:19