
Chemistry, 30.01.2021 07:30 jnsebastian2002
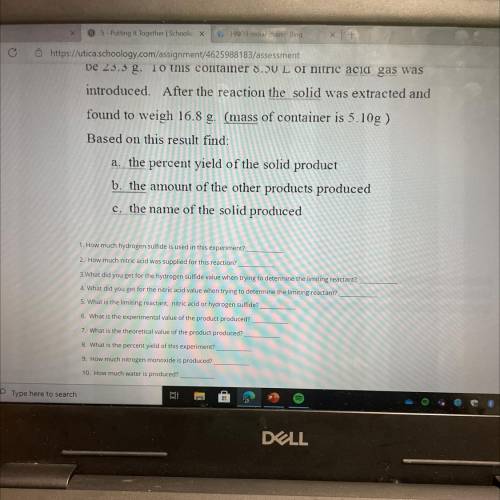
Elemental sulfur is one of the products of the gas-phase
reaction of nitric acid and hydrogen sulfide. The other
products are nitrogen monoxide(g) and water(g). A
container and hydrogen sulfide (s) are massed and found to
be 23.3 g. To this container 8.50 L of nitric acid gas was
introduced. After the reaction the solid was extracted and
found to weigh 16.8 g. (mass of container is 5.10g )
Based on this result find:
a. the percent yield of the solid product
b. the amount of the other products produced
c. the name of the solid produced
1 How much hydrogen sulfide is used in this experiment?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1

Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3

Chemistry, 22.06.2019 20:00
If one fission reaction of a uranium-235 atom produced two neutrons, how many neutrons would be released if the chain reaction occurred three more times?
Answers: 1

Chemistry, 22.06.2019 22:30
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1
You know the right answer?
Elemental sulfur is one of the products of the gas-phase
reaction of nitric acid and hydrogen...
reaction of nitric acid and hydrogen...
Questions

Mathematics, 07.12.2021 04:40

Chemistry, 07.12.2021 04:40


Arts, 07.12.2021 04:40

SAT, 07.12.2021 04:40


Mathematics, 07.12.2021 04:40


Mathematics, 07.12.2021 04:40



Mathematics, 07.12.2021 04:40

History, 07.12.2021 04:40

Mathematics, 07.12.2021 04:40


English, 07.12.2021 04:40

Geography, 07.12.2021 04:40






