
Chemistry, 30.01.2021 19:00 strawberrymrmr756
Write the equilibrium expression for the following reaction. based upon the value of k, would there be more or less product in the equilibrium mixture?why? CaO(s)+CH4(g)+2H2O(g) = CaCO3(s)+4H2(g) kp=2344

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:20
Determine which intermolecular forces are the dominant (strongest) forces for a pure sample of each of the following molecules by placing the molecules into the correct bins. drag the appropriate molecular formula to their respective bins.
Answers: 3

Chemistry, 21.06.2019 22:00
To save time, you can approximate the initial mass of the solid to the nearest ±1 g. for example, if you are asked to add 14.3 g of copper, add between 13 g and 15 g. which of the following sets include two samples with an equal density? which all that apply below 15.4 g gold and 18.7 g silver 15.2 g copper and 50.0 g copper 20.2 g silver and 20.2 g copper 11.2 g gold and 14.9 g gold
Answers: 1


You know the right answer?
Write the equilibrium expression for the following reaction. based upon the value of k, would there...
Questions

Mathematics, 27.09.2019 16:30


Mathematics, 27.09.2019 16:30

Biology, 27.09.2019 16:30

Mathematics, 27.09.2019 16:30


Biology, 27.09.2019 16:30


History, 27.09.2019 16:30

Arts, 27.09.2019 16:30


Mathematics, 27.09.2019 16:30

Chemistry, 27.09.2019 16:30


Mathematics, 27.09.2019 16:30

Health, 27.09.2019 16:30




Biology, 27.09.2019 16:30

![K_p=\frac{[H_2]^4}{[CH_4]^1[H_2O]^2}](/tpl/images/1080/2198/6177c.png)
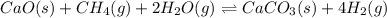
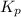 is written as:
is written as:

