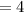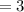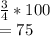Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Why did southern business leaders want to increase the number of slaves
Answers: 1

Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1

Chemistry, 22.06.2019 10:50
How many liters of oxygen gas, at standard temperature and pressure, will react with 35.8 grams of iron metal? 4 fe (s) + 3 o₂ (g) → 2 fe₂o₃ (s)
Answers: 2

Chemistry, 22.06.2019 14:00
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3
You know the right answer?
What is the percent/% yield if 4 moles of hydrogen is reacted with 3 moles
of oxygen and produces 3...
Questions


Arts, 29.01.2021 18:40




Mathematics, 29.01.2021 18:40


Mathematics, 29.01.2021 18:40

Mathematics, 29.01.2021 18:40

Mathematics, 29.01.2021 18:40

Mathematics, 29.01.2021 18:40



Mathematics, 29.01.2021 18:40


Mathematics, 29.01.2021 18:40

Computers and Technology, 29.01.2021 18:40


History, 29.01.2021 18:40

English, 29.01.2021 18:40

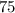 %
%