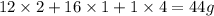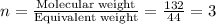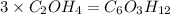Chemistry, 01.02.2021 14:00 ciaotaylor
A compound is determined to have the empirical formula C2OH4. If the molar mass of the compound is 132 g/mol, determine the molecular formula of the compound.


Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 22:20
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2

Chemistry, 22.06.2019 23:00
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1
You know the right answer?
A compound is determined to have the empirical formula C2OH4. If the molar mass of the compound is 1...
Questions

Mathematics, 27.02.2021 01:00

Mathematics, 27.02.2021 01:00



Mathematics, 27.02.2021 01:00

Mathematics, 27.02.2021 01:00

Chemistry, 27.02.2021 01:00

Social Studies, 27.02.2021 01:00

Mathematics, 27.02.2021 01:00


Mathematics, 27.02.2021 01:00

Mathematics, 27.02.2021 01:00


Mathematics, 27.02.2021 01:00

English, 27.02.2021 01:00


Mathematics, 27.02.2021 01:00


Geography, 27.02.2021 01:00

Mathematics, 27.02.2021 01:00

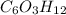
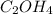 is
is