18
loose plug of cotton wool
dilute hydrochloric acid
.
Db
o a
o
magn...

Chemistry, 01.02.2021 15:50 littlesami105
18
loose plug of cotton wool
dilute hydrochloric acid
.
Db
o a
o
magnesium ribbon
Fiqure 6
The word equation for the reaction between magnesium and dilute hydrochloric acid is
magnesium + hydrochloric acid magnesium chloride + hydrogen
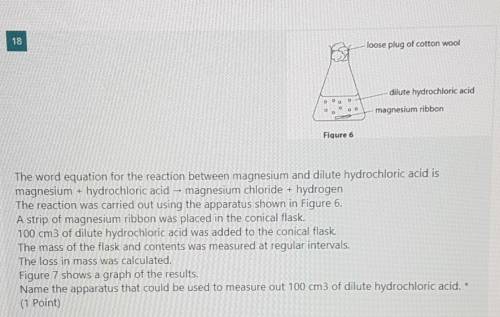
The reaction was carried out using the apparatus shown in Figure 6.
A strip of magnesium ribbon was placed in the conical flask.
100 cm3 of dilute hydrochloric acid was added to the conical flask.
The mass of the flask and contents was measured at regular intervals.
The loss in mass was calculated.
Figure 7 shows a graph of the results.
Name the apparatus that could be used to measure out 100 cm3 of dilute hydrochloric acid. *
(1 Point)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Which of the following compounds does not contain molecules? question 2 options: co2 h2 nacl h2o
Answers: 1

Chemistry, 22.06.2019 18:40
What is one real world example of a colligative property?
Answers: 2

Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3

You know the right answer?
Questions


Mathematics, 24.04.2020 17:27





English, 24.04.2020 17:27

Mathematics, 24.04.2020 17:27

Mathematics, 24.04.2020 17:27





French, 24.04.2020 17:28



Mathematics, 24.04.2020 17:28


Mathematics, 24.04.2020 17:28

Health, 24.04.2020 17:28



