20
20
RE
lin
DIG
DS
an
10000
na mim
Figure 1
The graph...

20
20
RE
lin
DIG
DS
an
10000
na mim
Figure 1
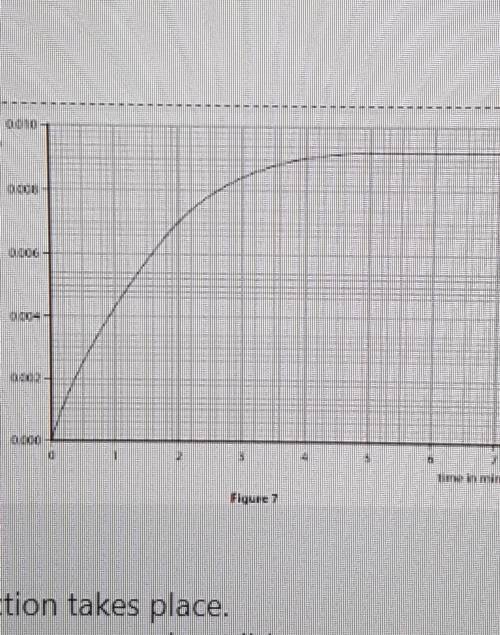
The graph shows that the rate of reaction slows as the reaction takes place.
Explain, in terms of particles, why the rate of reaction between magnesium ribbon and dilute
hydrochloric acid slows as the reaction takes place. M
(3 Points)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Based on the equation and the information in the table, what is the enthalpy of the reaction?
Answers: 2

Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 07:00
This image is an example of a(n) a) atom. b) compound. c) mixture. d) molecule.
Answers: 1

You know the right answer?
Questions

Biology, 11.03.2020 03:11


Mathematics, 11.03.2020 03:11

Physics, 11.03.2020 03:11










Mathematics, 11.03.2020 03:11






Mathematics, 11.03.2020 03:11



