
Chemistry, 01.02.2021 21:40 cjd1214812148
Problem 3 A sample of 2.37 moles of an ideal diatomic gas experiences a temperature increase of 65.2 K at constant volume. (a) Find the increase in internal energy if only translational and rotational motions are possible. (b) Find the increase in internal energy if translational, rotational, and vibrational motions are possible. (c) How much of the energy calculated in (a) and (b) is translational kinetic energy?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1


You know the right answer?
Problem 3 A sample of 2.37 moles of an ideal diatomic gas experiences a temperature increase of 65.2...
Questions

Social Studies, 10.03.2021 23:10



Mathematics, 10.03.2021 23:10




Mathematics, 10.03.2021 23:10


Mathematics, 10.03.2021 23:10

Mathematics, 10.03.2021 23:10





Mathematics, 10.03.2021 23:10

History, 10.03.2021 23:10

Mathematics, 10.03.2021 23:10

Mathematics, 10.03.2021 23:10

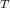 = 1927.06 J
= 1927.06 J

