
Many compressed gases come in large, heavy metal cylinders that are so bevy that they need a special cart to move them around. An 80.0-L tank of nitrogen gas pressurized to 172 arm atm is left in the sun and heats from its normal temperature of 20.0 degrees Celsius to 140.0 degrees Celsius. Determine (a) the final pressure inside the tank and (b) the work, heat, and delta U of the process. assume that behavior is ideal and the heat capacity of diatomic nitrogen is 21.0 j/molk.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:20
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3

Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1

Chemistry, 22.06.2019 17:30
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2
You know the right answer?
Many compressed gases come in large, heavy metal cylinders that are so bevy that they need a special...
Questions


History, 29.06.2019 08:20


Chemistry, 29.06.2019 08:20

Mathematics, 29.06.2019 08:20




Mathematics, 29.06.2019 08:20

Mathematics, 29.06.2019 08:20

Social Studies, 29.06.2019 08:20

English, 29.06.2019 08:20

Mathematics, 29.06.2019 08:20

Mathematics, 29.06.2019 08:30


Spanish, 29.06.2019 08:30

English, 29.06.2019 08:30

Advanced Placement (AP), 29.06.2019 08:30

Social Studies, 29.06.2019 08:30

Mathematics, 29.06.2019 08:30

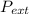 dv
dv


