
Chemistry, 02.02.2021 03:10 janellball16
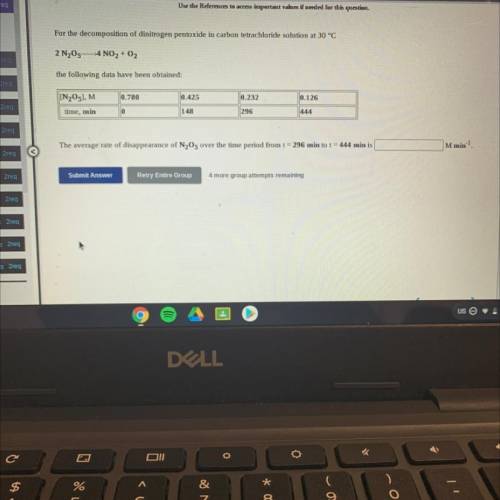
For the decomposition of dinitrogen pentoxide in carbon tetrachloride solution at 30 °C
2 N205-4 NO2 + O2
the following data have been obtained:
[N205], M
0.780
0.425
0.232
0.126
||444
time, min
0
148
296
M min-1
The average rate of disappearance of N2O5 over the time period from t = 296 min to t = 444 min is

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:40
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 23.06.2019 02:00
To calculate the molarity of a solution, you need to know the moles of solute and the
Answers: 2

Chemistry, 23.06.2019 03:00
What do electromagnetic waves carry? how are they produced through which media can they move? where do they transfer energy? what do they not transfer? what do mechanical waves carry? how are they produced? through which media can they move? where do they transfer energy? what do they not transfer?
Answers: 1
You know the right answer?
For the decomposition of dinitrogen pentoxide in carbon tetrachloride solution at 30 °C
2 N205-4 NO...
Questions



Mathematics, 11.01.2022 09:10

English, 11.01.2022 09:10

Mathematics, 11.01.2022 09:10


SAT, 11.01.2022 09:10

Mathematics, 11.01.2022 09:10

Mathematics, 11.01.2022 09:10

Advanced Placement (AP), 11.01.2022 09:10


Mathematics, 11.01.2022 09:10

Chemistry, 11.01.2022 09:10

Mathematics, 11.01.2022 09:10

Mathematics, 11.01.2022 09:10

Mathematics, 11.01.2022 09:20


Mathematics, 11.01.2022 09:20


Mathematics, 11.01.2022 09:20



