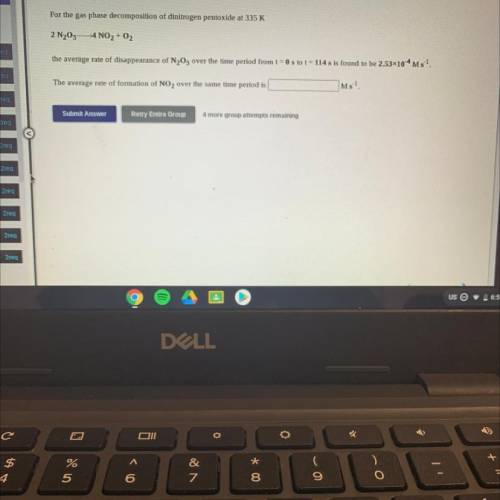
For the gas phase decomposition of dinitrogen pentoxide at 335 K
2 N205– 4 NO2 + O2
the avera...

For the gas phase decomposition of dinitrogen pentoxide at 335 K
2 N205– 4 NO2 + O2
the average rate of disappearance of N205 over the time period from t = 0 s to t = 114 s is found to be 2.53x10-4 M 5-1.
The average rate of formation of NO2 over the same time period is
Ms-1
Submit Answer
Retry Entire Group
4 more group attempts remaining

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 17:30
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1


Chemistry, 23.06.2019 06:00
When hydrogen peroxide (h2o2) is added to potassium iodide (ki) solution, the hydrogen peroxide decomposes into water (h2o) and oxygen (o2). the chemical equation for the decomposition reaction is: 2h2o2—> 2h2o + o2. what is the role of the potassium iodide in this reaction? a. reactant. b. product. c. precipitate. d. catalyst.
Answers: 1
You know the right answer?
Questions



Law, 10.11.2020 22:00

Mathematics, 10.11.2020 22:00

Mathematics, 10.11.2020 22:00

History, 10.11.2020 22:00

Chemistry, 10.11.2020 22:00

Biology, 10.11.2020 22:00

Advanced Placement (AP), 10.11.2020 22:00

Mathematics, 10.11.2020 22:00

Mathematics, 10.11.2020 22:00

Mathematics, 10.11.2020 22:00




History, 10.11.2020 22:00


Mathematics, 10.11.2020 22:00


Health, 10.11.2020 22:00



