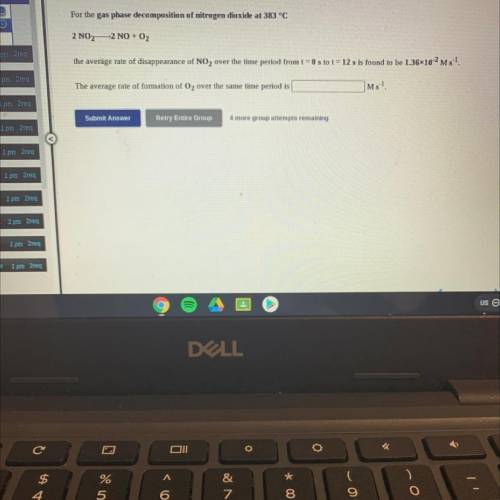
For the gas phase decomposition of nitrogen dioxide at 383 °C
2 NO2—2 NO + O2
the average rat...

Chemistry, 02.02.2021 03:20 tyneshiajones124
For the gas phase decomposition of nitrogen dioxide at 383 °C
2 NO2—2 NO + O2
the average rate of disappearance of NO2 over the time period from t = 0 s to t = 12 s is found to be 1.3610-2 Ms 1.
The average rate of formation of O2 over the same time period is
Ms-1

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 17:00
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1

Chemistry, 22.06.2019 19:20
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3
You know the right answer?
Questions



Mathematics, 11.07.2019 12:00


Mathematics, 11.07.2019 12:00

English, 11.07.2019 12:00

Social Studies, 11.07.2019 12:00

Mathematics, 11.07.2019 12:00


Mathematics, 11.07.2019 12:00


Mathematics, 11.07.2019 12:00


Mathematics, 11.07.2019 12:00

Mathematics, 11.07.2019 12:00

Mathematics, 11.07.2019 12:00

Mathematics, 11.07.2019 12:00

Chemistry, 11.07.2019 12:00

Geography, 11.07.2019 12:00

English, 11.07.2019 12:00



