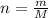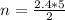Chemistry, 02.02.2021 04:00 ExperimentsDIYS
2. The heat from an acetylene torch is produced by burning acetylene (C2H2) in oxygen.
2C2H2 + 502 --> 4CO2 + 2H20
When 2.40mol C2H2 reacts with 7.40mol O2,
a. Which reactant is the limiting reactant?
b. How many grams of water can be produced by the reaction?
c. How many moles of the excess reactant remains?
I NEED THIS ANSWER QUICKLY IF YOU CAN ONLY DO B THATS FINE THANK YOU

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Select the correct answer. given: 2libr + ba → babr2 + 2li in this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced? a. 1.18 mol b. 2.37 mol c. 4.73 mol d. 16.4 mol e. 32.9 mol
Answers: 2

Chemistry, 22.06.2019 05:30
The climate of the continental united states is generally 1. tropical 2. temperate 3. arctic 4. highland
Answers: 1

Chemistry, 22.06.2019 08:30
Which metal exist in liquid state and can be cut with knife ?
Answers: 2

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1
You know the right answer?
2. The heat from an acetylene torch is produced by burning acetylene (C2H2) in oxygen.
2C2H2 + 502...
Questions

Social Studies, 13.12.2019 08:31

Mathematics, 13.12.2019 08:31

Social Studies, 13.12.2019 08:31


Social Studies, 13.12.2019 08:31


Social Studies, 13.12.2019 08:31


Mathematics, 13.12.2019 08:31

Mathematics, 13.12.2019 08:31

Mathematics, 13.12.2019 08:31






English, 13.12.2019 08:31

Mathematics, 13.12.2019 08:31


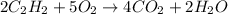
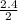 = 1.2
= 1.2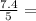 1.48
1.48