
Chemistry, 02.02.2021 04:20 jaystitt1152683
Methyl isocyanate, shown as resonance structure 1, can also be represented by other resonance structures. Draw the next most important resonance contributor. Then add curved arrows to each structure to show delocalization of electron pairs to form the other structure. Include lone pairs of electrons, formal charges, and hydrogen atoms. You can add condensed hydrogens using the More menu, selecting +H and clicking on the carbon as many times as needed.

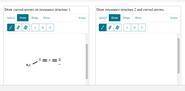

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Why are people not able to scuba dive in the deep part of the ocean
Answers: 2

Chemistry, 22.06.2019 07:00
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 07:30
Plz mark brainliest 30 points 1) find the momentum of a 12 kg snowball that is rolling with a velocity of 9 m/s. 2) an 8 ball with a mass of .5 kg is sitting at rest. it is hit by the cue ball (1 kg) traveling at 2.5 m/s. if the cue ball is at rest after the collision, how fast is the 8 ball traveling after the collision? 3) two football players are running toward each other. if the offensive player is 75 kg and is running 8 m/s, how fast must the 60 kg defensive player run in order for the two players to hit and stop?
Answers: 1

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1
You know the right answer?
Methyl isocyanate, shown as resonance structure 1, can also be represented by other resonance struct...
Questions

Mathematics, 28.01.2020 02:31





History, 28.01.2020 02:31


Mathematics, 28.01.2020 02:31

Social Studies, 28.01.2020 02:31

Mathematics, 28.01.2020 02:31

Mathematics, 28.01.2020 02:31

Mathematics, 28.01.2020 02:31

History, 28.01.2020 02:31


History, 28.01.2020 02:31


Mathematics, 28.01.2020 02:31

Spanish, 28.01.2020 02:31


History, 28.01.2020 02:31



