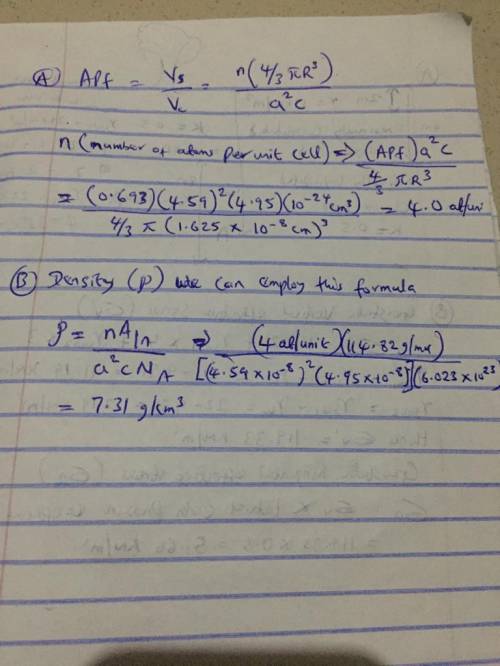Chemistry, 02.02.2021 05:50 ob3ymysins
Indium has a tetragonal unit cell for which the a and c lattice parameters are 0.459 and 0.495 nm, respectively. (a) If the atomic packing factor and atomic radius are 0.693 and 0.1625 nm, respectively, determine the number of atoms in each unit cell. (b) The atomic weight of indium is 114.82 g/mol; compute its theoretical density. (a) Enter your answer for part (a) in accordance to the question statement atoms/unit cell (b) Enter your answer for part (b) in accordance to the question statement g/cm3

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2

You know the right answer?
Indium has a tetragonal unit cell for which the a and c lattice parameters are 0.459 and 0.495 nm, r...
Questions

English, 05.05.2020 14:03

Mathematics, 05.05.2020 14:03


Biology, 05.05.2020 14:03




Biology, 05.05.2020 14:03



Mathematics, 05.05.2020 14:03

Mathematics, 05.05.2020 14:03

Biology, 05.05.2020 14:03

Mathematics, 05.05.2020 14:03




Mathematics, 05.05.2020 14:03

English, 05.05.2020 14:03