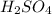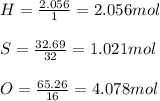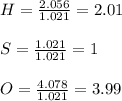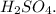Chemistry, 02.02.2021 14:00 adamkinney6110
A compound was analyzed and found to contain the following percent composition: 2.056% hydrogen, 32.69% S, and 65.26% oxygen. Calculate the empirical formula.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
If i make a solution by adding 83grams of sodium hydroxide to 750ml i’d water what is the molarity of sodium hydroxide
Answers: 1

Chemistry, 21.06.2019 22:00
If a plot weight (in g) vs. volume (in ml) for a metal gave the equation y= 13.41x and r^2=0.9981 what is the density of the metal?
Answers: 2

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 15:20
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1
You know the right answer?
A compound was analyzed and found to contain the following percent composition: 2.056% hydrogen, 32....
Questions

Mathematics, 02.06.2021 18:00


Social Studies, 02.06.2021 18:00

Mathematics, 02.06.2021 18:00



History, 02.06.2021 18:00




Mathematics, 02.06.2021 18:00

Mathematics, 02.06.2021 18:00

Mathematics, 02.06.2021 18:00



Mathematics, 02.06.2021 18:00



Mathematics, 02.06.2021 18:00