
Chemistry, 02.02.2021 16:50 daeshawnc14
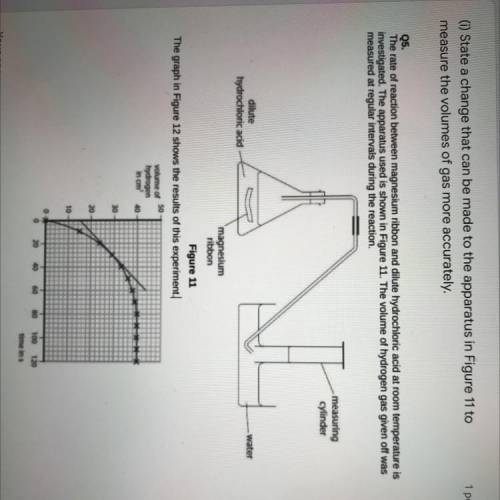
(1)State a change that can be made to the apparatus in Figure 11 to
measure the volumes of gas more accurately.
Q5.
The rate of reaction between magnesium ribbon and dilute hydrochloric acid at room temperature is
investigated. The apparatus used is shown in Figure 11. The volume of hydrogen gas given off was
measured at regular intervals during the reaction.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

Chemistry, 22.06.2019 10:40
Asolid that forms and separates from a liquid mixture is called
Answers: 2

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1
You know the right answer?
(1)State a change that can be made to the apparatus in Figure 11 to
measure the volumes of gas more...
Questions






Mathematics, 24.03.2020 16:59


Mathematics, 24.03.2020 16:59

Mathematics, 24.03.2020 16:59



Mathematics, 24.03.2020 16:59










