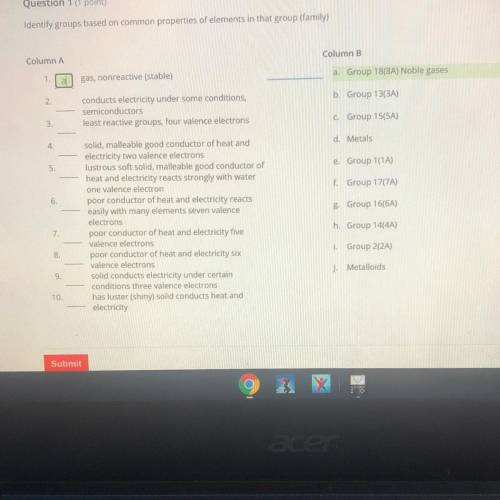
Column A
Column B
a
gas, nonreactive (stable)
a. Group 18(8A) Noble gases
2...

Chemistry, 02.02.2021 20:10 liyahmakay1853
Column A
Column B
a
gas, nonreactive (stable)
a. Group 18(8A) Noble gases
2.
b. Group 13(3A)
conducts electricity under some conditions,
semiconductors
least reactive groups, four valence electrons
3
C Group 15(5A)
4
d. Metals
5
e. Group 1(1A)
f. Group 177A)
6
g Group 16(6A)
solid, malleable good conductor of heat and
electricity two valence electrons
lustrous soft solid, malleable good conductor of
heat and electricity reacts strongly with water
one valence electron
poor conductor of heat and electricity reacts
easily with many elements seven valence
electrons
poor conductor of heat and electricity five
valence electrons
poor conductor of heat and electricity six
valence electrons
solid conducts electricity under certain
conditions three valence electrons
has luster (shiny) solid conducts heat and
electricity
7
h. Group 14(4A)
8
i. Group 2(2A)
j. Metalloids
9.
10.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
What is the result of multiplying (2.5 × 1010) × (2.0 × 10-7)? a. 5.0 × 103 b. 5.0 × 10-3 c. 5.0 × 1017 d. 5.0 × 10-17
Answers: 1

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3

Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1
You know the right answer?
Questions






















