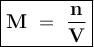Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 22:10
How do forces between particles in gases compare to forces in the other states of matter? o a. the forces in gases are stronger than forces in solids but weaker than forces in liquids. o b. the forces in gases are weaker than forces in solids but stronger than forces in liquids. o c. the forces in gases are weaker than forces in solids and liquids. o d. the forces in gases are stronger than forces in solids and liquids. submit
Answers: 1

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

You know the right answer?
The molecular weight of KMnO4 is 158 g/mol. If you wanted to make 500 mL of a 0.1M stock solution of...
Questions




Mathematics, 21.05.2020 21:02

English, 21.05.2020 21:02


Mathematics, 21.05.2020 21:02








Mathematics, 21.05.2020 21:03