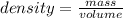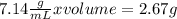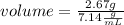Chemistry, 03.02.2021 05:10 NikkiZoeller
A 2.67 g piece of zinc (density = 7.14 g/mL) is added to a graduated cylinder that contains 12.13 mL H 2 O . What will be the final volume reading on the graduated cylinder, in mL?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Rocks, as they are compressed, begin forming mountains above the earth's surface when two continental plates converge. the continental crust increases in depth as the mountains grow above. the himalayan mountains formed at a convergent plate boundary in this manner. the rocks are smashed together causing them to due to the intense heat and pressure from the colliding plates and eventually forming rock. a) melt; igneous b) layer; sedimentary c) recrystallize; metamorphic d) melt into the earth's interior; metamorphic
Answers: 1

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1

Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3
You know the right answer?
A 2.67 g piece of zinc (density = 7.14 g/mL) is added to a graduated cylinder that contains 12.13 mL...
Questions


Mathematics, 18.02.2021 22:40



Mathematics, 18.02.2021 22:40

Mathematics, 18.02.2021 22:40

Mathematics, 18.02.2021 22:40


English, 18.02.2021 22:40

Biology, 18.02.2021 22:40


Spanish, 18.02.2021 22:40

Social Studies, 18.02.2021 22:40

Mathematics, 18.02.2021 22:40


Mathematics, 18.02.2021 22:40



Physics, 18.02.2021 22:40