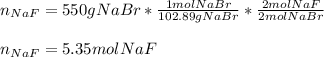Chemistry, 03.02.2021 06:30 ReveenatheRaven2296
How many moles of NaF are produced in the reaction between sodium bromide and calcium fluoride when 550 grams
of sodium bromide are used
2NaBr+CaF2 -> 2NaF+CaBr2
A.0.53 mol NaF
B. 5g NaF
C. 10.7 mol NaF
D. 5.35 g NaF

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3

Chemistry, 22.06.2019 22:30
What is the value of the standard enthalpy of formation of an element in its most stable form?
Answers: 3
You know the right answer?
How many moles of NaF are produced in the reaction between sodium bromide and calcium fluoride when...
Questions



Mathematics, 02.03.2021 07:30

Mathematics, 02.03.2021 07:30






Mathematics, 02.03.2021 07:30

History, 02.03.2021 07:30

Mathematics, 02.03.2021 07:30

Business, 02.03.2021 07:30

Mathematics, 02.03.2021 07:30

Biology, 02.03.2021 07:30

Mathematics, 02.03.2021 07:30


Mathematics, 02.03.2021 07:30


Chemistry, 02.03.2021 07:30