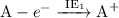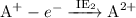Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1

Chemistry, 22.06.2019 14:00
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 23.06.2019 03:00
Is it safe to take 450mg of diphenhydramine hydrochloride?
Answers: 1
You know the right answer?
Among the elements of the main group, the first ionization energy increases
a. from left to ri...
a. from left to ri...
Questions



Computers and Technology, 08.01.2021 01:30

Arts, 08.01.2021 01:30

Mathematics, 08.01.2021 01:30

Physics, 08.01.2021 01:30


Mathematics, 08.01.2021 01:30




History, 08.01.2021 01:30

Physics, 08.01.2021 01:40

Health, 08.01.2021 01:40

History, 08.01.2021 01:40

Mathematics, 08.01.2021 01:40

Mathematics, 08.01.2021 01:40

Mathematics, 08.01.2021 01:40

Social Studies, 08.01.2021 01:40

Mathematics, 08.01.2021 01:40

 .
.