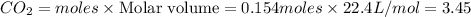Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:40
If 10.0 ml of the solution on the right are withdrawn from the 100 ml beaker and diluted again in a similar manner, what is the new concentration? m nacl
Answers: 2

Chemistry, 22.06.2019 03:30
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 15:50
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1

You know the right answer?
C3H8 + 5O2 → 3CO2+ 4H2O, if 5.75L of oxygen are consumed in the above reaction, how many L of carbon...
Questions

History, 08.12.2019 04:31




Mathematics, 08.12.2019 04:31

Physics, 08.12.2019 04:31

Mathematics, 08.12.2019 04:31






Mathematics, 08.12.2019 04:31

Mathematics, 08.12.2019 04:31

Social Studies, 08.12.2019 04:31

Mathematics, 08.12.2019 04:31



History, 08.12.2019 04:31

Mathematics, 08.12.2019 04:31

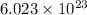 of particles.
of particles.
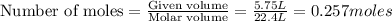
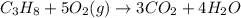
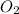 produce = 3 moles of
produce = 3 moles of 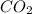
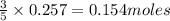 of
of