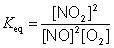Chemistry, 04.02.2021 14:00 kayden1234thomp
Identify the correct equilibrium constant expression for this equation:
2 upper N upper O (g) plus upper O subscript 2 (g) double-headed arrow 2 upper N upper O subscript 2 (g).
K subscript e q equals StartFraction StartBracket upper N upper O EndBracket superscript 2 StartBracket upper O subscript 2 EndBracket over StartBracket upper N upper O EndBracket superscript 2 EndFraction.
K subscript e q equals StartFraction StartBracket upper N upper O subscript 2 EndBracket superscript 2 over StartBracket upper N upper O EndBracket superscript 2 StartBracket upper O subscript 2 EndBracket EndFraction.
K subscript e q equals StartFraction StartBracket upper N upper O EndBracket over StartBracket upper N Upper O subscript 2 EndBracket StartBracket upper O subscript 2 EndBracket EndFraction.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3
You know the right answer?
Identify the correct equilibrium constant expression for this equation:
2 upper N upper O (g) plus...
Questions

Mathematics, 14.12.2021 05:10

Mathematics, 14.12.2021 05:10

English, 14.12.2021 05:10


Social Studies, 14.12.2021 05:10




Mathematics, 14.12.2021 05:10





Mathematics, 14.12.2021 05:10



Mathematics, 14.12.2021 05:10


Social Studies, 14.12.2021 05:10