
Energy Efficiency Practice
Efficiency is the (often measurable) ability to avoid wasting materials, energy, efforts, money, and time in doing
something or in producing a desired result in a more general sense, it is the ability to do things well,
successfully, and without waste. Efficiency is very often confused with effectiveness. In general, efficiency is a
measurable concept, quantitatively determined by the ratio of useful output to total useful input. Effectiveness
is the simpler concept of being able to achieve a desired result, which can be expressed quantitatively but
does not usually require more complicated mathematics than addition,
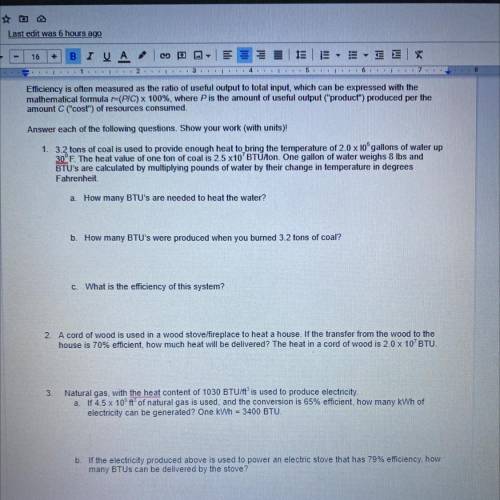
Efficiency is often measured as the ratio of useful output to total input, which can be expressed with the
mathematical formula r=(PIC) x 100%, where P is the amount of useful output ("product") produced per the
amount C ("cost") of resources consumed.
Answer each of the following questions. Show your work (with units)!
1. 3.2 tons of coal is used to provide enough heat to bring the temperature of 2.0 x 10 gallons of water up
30°F. The heat value of one ton of coal is 2.5 x10' BTU/on. One gallon of water weighs 8 lbs and
BTU's are calculated by multiplying pounds of water by their change in temperature in degrees
Fahrenheit
a. How many BTU's are needed to heat the water?
b. How many BTU's were produced when you burned 3.2 tons of coal?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Nconcentration refers to the molar concentration of an ion in solution. it may be identical to, or greater or less than, the molar concentration of the compound containing the ion that was used to make the solution. for soluble salts, the molarity of a particular ion is equal to the molarity of that compound times the subscript for that ion. for example, 1 m of alcl3 is 1 m in al3+ and 3 m in cl−. 1 m of (nh4)2so4 is 2 m in nh4+ and 1 m in so42−. part a what is the concentration of k+ in 0.15 m of k2s? view available hint(s) nothing m m part b if cacl2 is dissolved in water, what can be said about the concentration of the ca2+ ion? view available hint(s) if is dissolved in water, what can be said about the concentration of the ion? it has the same concentration as the cl− ion. its concentration is half that of the cl− ion. its concentration is twice that of the cl− ion. its concentration is one-third that of the cl− ion. part c a scientist wants to make a solution of tribasic sodium phosphate, na3po4, for a laboratory experiment. how many grams of na3po4 will be needed to produce 550 ml of a solution that has a concentration of na+ ions of 0.700 m ? express your answer numerically in grams. view available hint(s) mass of na3po4 n a 3 p o 4 = nothing g provide feedback
Answers: 3

Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

Chemistry, 22.06.2019 13:00
These questions are based on the attached photo. the experiment is about burning magnesium metal with oxygen. 1. write the balanced chemical equation for the reaction you are performing. 2. calculate the mass of magnesium metal used in each trial. o trial 1: o trial 2: 3. calculate the actual yield of magnesium oxide for each trial. o trial 1: o trial 2: 4. magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. o trial 1: o trial 2: 5. determine the percent yield of mgo for your experiment for each trial. o trial 1: o trial 2: 6. determine the average percent yield of mgo for the two trials. your company currently uses a process with a similar cost of materials that has an average percent yield of 91 percent. if the average percent yield of this process is higher than that, this could save the company money. what is your recommendation to the company? support your recommendation using your data, calculations, and understanding of stoichiometry gathered from this lab.
Answers: 1

Chemistry, 23.06.2019 03:20
High-pressure liquid chromatography (hplc) is a method used in chemistry and biochemistry to purify chemical substances. the pressures used in this procedure range from around 500 kilopascals (500,000 pa) to about 60,000 kpa (60,000,000 pa). it is often convenient to know the pressure in torr. if an hplc procedure is running at a pressure of 1.03×108 pa , what is its running pressure in torr?
Answers: 3
You know the right answer?
Energy Efficiency Practice
Efficiency is the (often measurable) ability to avoid wasting materials,...
Questions




Mathematics, 25.07.2019 15:50

Mathematics, 25.07.2019 15:50

Mathematics, 25.07.2019 15:50



Social Studies, 25.07.2019 15:50


Chemistry, 25.07.2019 15:50




Biology, 25.07.2019 16:00







