Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
The diagram shows the structures of horse and cat forelimbs. what does the diagram suggest about the evolutionary relationship between these two mammals? a. they have homologous structures, indicating a common ancestor. b. they have analogous structures, indicating a common ancestor. c. they have homologous structures, indicating that they do not have a common ancestor. d. they have analogous structures, indicating that they do not have a common ancestor.
Answers: 2

Chemistry, 22.06.2019 01:00
The diagram shows the positions of the sun, moon and earth during spring tides, when the high tides are at their highest and low tides at their lowest. what is it about these positions that causes these high and low tides?
Answers: 3

Chemistry, 22.06.2019 07:30
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1
You know the right answer?
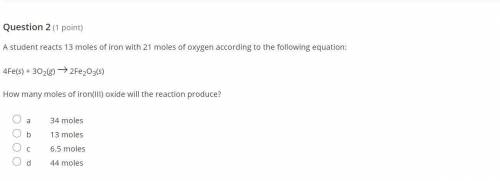
A student reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
4Fe(...
Questions

History, 06.10.2020 05:01

Mathematics, 06.10.2020 05:01




Mathematics, 06.10.2020 05:01

Mathematics, 06.10.2020 05:01

Spanish, 06.10.2020 05:01

History, 06.10.2020 05:01



History, 06.10.2020 05:01



French, 06.10.2020 05:01



Business, 06.10.2020 05:01

Mathematics, 06.10.2020 05:01