
Chemistry, 05.02.2021 09:50 dontcareanyonemo
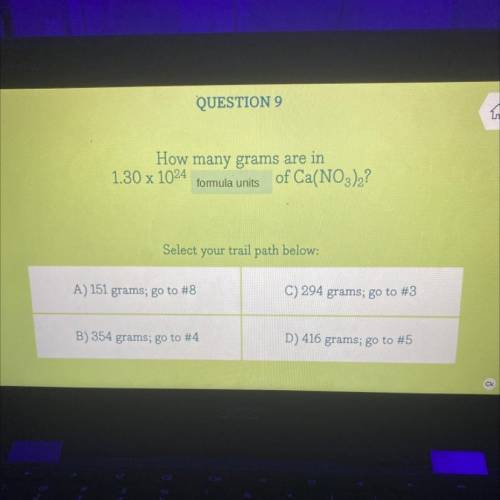
How many grams are in 1.30 x 10^24 formula units of Ca(NO3)2?
A. 151 g
B. 354 g
C. 294 g
D. 416 g

Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 20:30
What problem would a person have if the nucleic acid in one of his or her cells were damaged?
Answers: 2

Chemistry, 21.06.2019 21:30
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10^-3 m and k for the dissociation is 1.86x10^-5. ch3cooh(aq)+h2o(> h3o^+(aq)+ch3coo^-(aq)
Answers: 2

Chemistry, 22.06.2019 07:00
This image is an example of a(n) a) atom. b) compound. c) mixture. d) molecule.
Answers: 1
You know the right answer?
How many grams are in 1.30 x 10^24 formula units of Ca(NO3)2?
A. 151 g
B. 354 g
C. 294...
B. 354 g
C. 294...
Questions


Mathematics, 06.11.2019 06:31

Biology, 06.11.2019 06:31


Mathematics, 06.11.2019 06:31


Health, 06.11.2019 06:31

Mathematics, 06.11.2019 06:31

Physics, 06.11.2019 06:31

History, 06.11.2019 06:31

History, 06.11.2019 06:31

Mathematics, 06.11.2019 06:31

History, 06.11.2019 06:31



Mathematics, 06.11.2019 06:31


Mathematics, 06.11.2019 06:31


Mathematics, 06.11.2019 06:31



