
Consider this reaction: 2so2 (g) + o2 (g) 2so3 (g) + heat. what will happen if more sulfur trioxide gas is added to the reaction mixture?
a-the equilibrium will shift toward the products.
b-the equilibrium will shift toward the reactants.
c-keq will increase.
d-keq will decrease.
e-there will be no shift in equilibrium.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:30
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 22.06.2019 12:00
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1


Chemistry, 22.06.2019 21:30
While in europe, if you drive 125 km per day, how much money would you spend on gas in one week if gas costs 1.10 euros per liter and your car’s gas mileage is 32.0 mi/gal? assume that 1 euro=1.26 dollars
Answers: 2
You know the right answer?
Consider this reaction: 2so2 (g) + o2 (g) 2so3 (g) + heat. what will happen if more sulfur trioxide...
Questions

Health, 06.01.2020 18:31



Physics, 06.01.2020 18:31







Social Studies, 06.01.2020 18:31






Business, 06.01.2020 18:31



Health, 06.01.2020 18:31

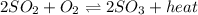
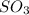 is added
is added

