
Chemistry, 05.02.2021 20:20 youngsavage10120
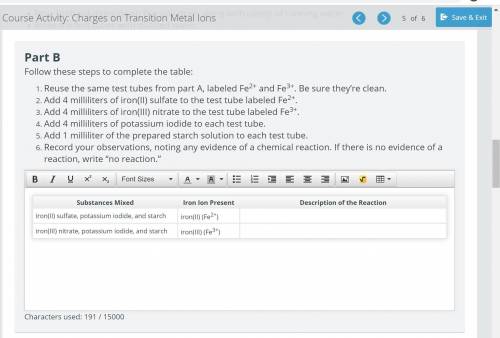
Reuse the same test tubes from part A, labeled Fe2+ and Fe3+. Be sure they’re clean.
Add 4 milliliters of iron(II) sulfate to the test tube labeled Fe2+.
Add 4 milliliters of iron(III) nitrate to the test tube labeled Fe3+.
Add 4 milliliters of potassium iodide to each test tube.
Add 1 milliliter of the prepared starch solution to each test tube.
Record your observations, noting any evidence of a chemical reaction. If there is no evidence of a reaction, write “no reaction.”

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 06:00
If you burn 10 kilograms of wood in a fire (combustion) what is the weight of the products after the fire has finished burning the wood?
Answers: 3

Chemistry, 22.06.2019 20:00
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1

Chemistry, 23.06.2019 00:30
•hydration •dissociation •dissolving which one goes to which
Answers: 1
You know the right answer?
Reuse the same test tubes from part A, labeled Fe2+ and Fe3+. Be sure they’re clean.
Add 4 millilit...
Questions

Physics, 15.07.2019 04:30


Mathematics, 15.07.2019 04:30

Biology, 15.07.2019 04:30


Business, 15.07.2019 04:30

Mathematics, 15.07.2019 04:30

Biology, 15.07.2019 04:30


History, 15.07.2019 04:30

Social Studies, 15.07.2019 04:30

Mathematics, 15.07.2019 04:30



Mathematics, 15.07.2019 04:30





English, 15.07.2019 04:30



