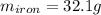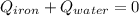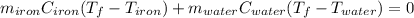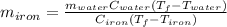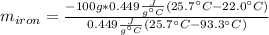Chemistry, 05.02.2021 21:20 eeeeee7891
A sample of iron, which has a specific heat capacity of 0.449 Jg^-1℃^-1, is put into a calorimeter (see sketch at right) that contains 100.0 g of water. The iron sample starts off at container 93.3 °C and the temperature of the water starts off at 22.0 °C. When the temperature of the water stops changing it's 25.7 °C. The pressure remains constant at 1 atm.
Required:
Calculate the mass of the iron sample. Be sure your answer is rounded to 2 significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 07:00
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1

Chemistry, 22.06.2019 09:00
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2

Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3
You know the right answer?
A sample of iron, which has a specific heat capacity of 0.449 Jg^-1℃^-1, is put into a calorimeter (...
Questions


Mathematics, 14.03.2020 02:27


Arts, 14.03.2020 02:28


Mathematics, 14.03.2020 02:28






Mathematics, 14.03.2020 02:28


Mathematics, 14.03.2020 02:28

Mathematics, 14.03.2020 02:28

Mathematics, 14.03.2020 02:28

Social Studies, 14.03.2020 02:28

English, 14.03.2020 02:28