
Chemistry, 05.02.2021 22:00 catsareokiguess
A solid sample of Zinc Hydroxide is added to 0.350 L of 0.500 M aqueous Hydrogen Bromide. The solution that remains is still acidic. It is then titrated with 0.500 M NaOH solution, and it takes 88.5 mL of the NaOH solution to reach the equivalence point. What mass of Zinc Hydroxide was added to the Hydrogen Bromide solution?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:20
What is the strongest intermolecular force between an nacl unit and an h2o molecule together in a solution? covalent bonding dipole-dipole force hydrogen bonding ion-dipole force
Answers: 1

Chemistry, 22.06.2019 07:30
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1

Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1

Chemistry, 23.06.2019 00:00
Mercury turns to a vapor at 629.88 k. how much heat is lost when 75.0 g of mercury vapor at 650 k condenses to a liquid at 297 k?
Answers: 1
You know the right answer?
A solid sample of Zinc Hydroxide is added to 0.350 L of 0.500 M aqueous Hydrogen Bromide. The soluti...
Questions

Mathematics, 20.09.2020 03:01

Mathematics, 20.09.2020 03:01


English, 20.09.2020 03:01


Biology, 20.09.2020 03:01





Spanish, 20.09.2020 03:01


Mathematics, 20.09.2020 03:01

Mathematics, 20.09.2020 03:01

Biology, 20.09.2020 03:01

Mathematics, 20.09.2020 03:01




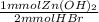 = 65.375 mmol Zn(OH)₂.
= 65.375 mmol Zn(OH)₂.

