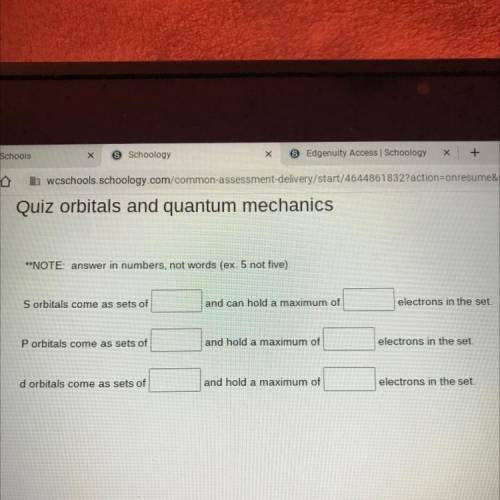
**NOTE: answer in numbers, not words (ex. 5 not five)
Sorbitals come as sets of
and can hold...

**NOTE: answer in numbers, not words (ex. 5 not five)
Sorbitals come as sets of
and can hold a maximum of
electrons in the set.
P orbitals come as sets of
and hold a maximum of
electrons in the set.
d orbitals come as sets of
and hold a maximum of
electrons in the set.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:10
Answer from each drop-down menu. e characteristics of a borane molecule (bh). the lewis structure and table of electronegativities are given olecular shape is and the molecule is reset next erved. search e a
Answers: 2

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 22.06.2019 17:10
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1

Chemistry, 22.06.2019 22:30
Which of the following molecules is polar? c3h7oh c2h5cooh
Answers: 1
You know the right answer?
Questions


Computers and Technology, 01.02.2021 16:30



Social Studies, 01.02.2021 16:30

Mathematics, 01.02.2021 16:30


History, 01.02.2021 16:30




English, 01.02.2021 16:30


Mathematics, 01.02.2021 16:30

Mathematics, 01.02.2021 16:30

Health, 01.02.2021 16:30

Physics, 01.02.2021 16:30

Mathematics, 01.02.2021 16:30


Mathematics, 01.02.2021 16:30



