Chemistry, 06.02.2021 02:00 sarah19Nursing
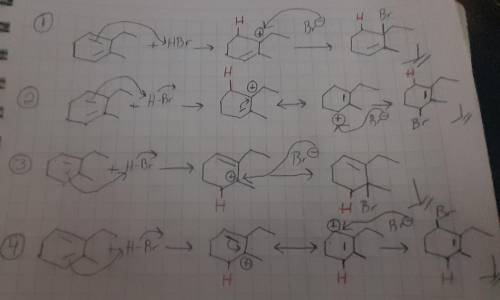
Draw all four products obtained when 2-ethyl-3-methyl-1,3-cyclohexadiene is treated with HBr at room temperature and show the mechanism of their formation. For the mechanism, include lone pairs and charges in your answer. Do not draw out any hydrogen explicitly. Do not use abbreviations such as Me or Ph.

Answers: 2


Another question on Chemistry


Chemistry, 23.06.2019 03:00
Use the half-reactions of the reaction au(oh)3 + hi -> au +i2 +h2o to answer the questions
Answers: 1

Chemistry, 23.06.2019 04:31
How does a sample of helium at 15 degree celsius compare to a sample of helium at 215 k? a) the helium at 15 degrees celsius has a higher average kinetic energy that the sample at 215 k. b) the helium at 15 degrees celsius has lower nuclear energy that the sample at 215 k. c) the helium at 15 degrees celsius has slower- moving atoms that the sample at 215 k. d) the helium at 15 degrees celsius has smaller atoms than the sample at 215 k.
Answers: 1

You know the right answer?
Draw all four products obtained when 2-ethyl-3-methyl-1,3-cyclohexadiene is treated with HBr at room...
Questions

Mathematics, 03.05.2020 13:06

Computers and Technology, 03.05.2020 13:06

Health, 03.05.2020 13:06



Mathematics, 03.05.2020 13:06


Mathematics, 03.05.2020 13:06

Mathematics, 03.05.2020 13:06


English, 03.05.2020 13:06

History, 03.05.2020 13:06


English, 03.05.2020 13:06

English, 03.05.2020 13:06

Mathematics, 03.05.2020 13:06


Mathematics, 03.05.2020 13:06

English, 03.05.2020 13:06

Mathematics, 03.05.2020 13:06