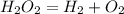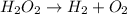Chemistry, 06.02.2021 02:20 abelxoconda
A 8.15 g sample of hydrogen peroxide (H2O2) decomposes to form water and oxygen. The temperature and pressures conditions in the lab were 21.2oC and 761.4 torr, respectively. The oxygen gas is collected over a sample of water at 21.2oC; the vapor pressure of water at that temperature is 18.9 torr. When the water level inside and outside of the tube is equal the volume of gas is recorded as 176.23 mL. a) Write and balance the equation for the decomposition reaction

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which feature do highland climates have that lower elevation areas do not?
Answers: 1

Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 14:30
Amixture that has two or more substances that are spread out evenly is called a. compound b. heterogeneous c. substance d. homogeneous
Answers: 1
You know the right answer?
A 8.15 g sample of hydrogen peroxide (H2O2) decomposes to form water and oxygen. The temperature and...
Questions

Chemistry, 23.07.2019 02:00

Health, 23.07.2019 02:00





Biology, 23.07.2019 02:00

Health, 23.07.2019 02:00


Social Studies, 23.07.2019 02:00


Chemistry, 23.07.2019 02:00

Social Studies, 23.07.2019 02:00





Mathematics, 23.07.2019 02:00

History, 23.07.2019 02:00