Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 21:00
Write a balanced equation showing the formation of copper (ii) nitrite from its elements
Answers: 1

Chemistry, 22.06.2019 22:30
What relationship exists between an enzyme and a catalyst?
Answers: 1

Chemistry, 23.06.2019 05:50
Which of the following isotopes has the same number of neutrons as phosphorus-31?
Answers: 1

Chemistry, 23.06.2019 06:40
4786 joules of heat are transferred to a 89.0 gramsample of an unknown material, with an initialtemperature of 23.0°c. what is the specific heat of thematerialif the final temperature is 89.5 °c?
Answers: 1
You know the right answer?
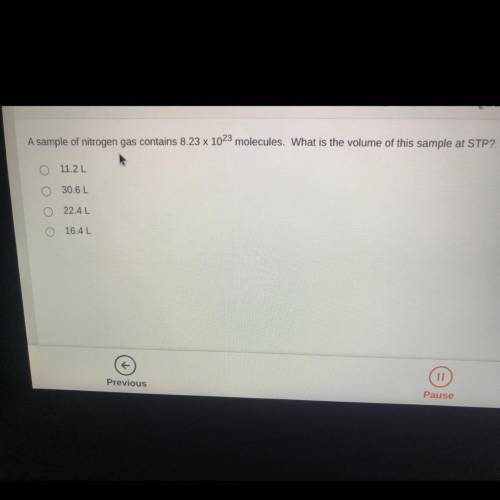
A sample of nitrogen gas contains 8.23 x 10^23 molecules. What is the volume of this sample at STP?...
Questions


Chemistry, 21.01.2020 05:31

Social Studies, 21.01.2020 05:31

History, 21.01.2020 05:31

Mathematics, 21.01.2020 05:31


Chemistry, 21.01.2020 05:31






Mathematics, 21.01.2020 05:31

Mathematics, 21.01.2020 05:31




Biology, 21.01.2020 05:31

English, 21.01.2020 05:31

Biology, 21.01.2020 05:31