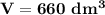Chemistry, 06.02.2021 05:40 tylerbrewton23
The reaction A→B is to be carried out isothermally in a continuous-flow reactor. Calculate the PFR volume to consume 99% of A (CA = 0.01CA0) when the entering molar flow rate is 5 mol/h (assume pure A), the volumetric flow rate is constant at 10 dm3 /h and the rate is: -rA=3CA 2 [ dm3 /mol•h]

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3

Chemistry, 23.06.2019 07:40
Which of the following has expanded our knowledge of the universe beyond our solar system the most? a. manned space travel b. the hubble space telescope c. the pioneer and voyager missions d. the international space station
Answers: 3

Chemistry, 23.06.2019 08:30
Of the following elements, which is the least reactive? a. c b. h c. li d. he
Answers: 1

Chemistry, 23.06.2019 14:20
Compounds a and b react to form compounds c and d according to the equation: aa + bb → cc + dd. under which conditions will the rate law be given by the equation: rate = k[a]a[b]b? a. the reaction takes place in one step. b. the reaction is endothermic. c. the reaction is exothermic. d. the reaction involves more than one step.
Answers: 3
You know the right answer?
The reaction A→B is to be carried out isothermally in a continuous-flow reactor. Calculate the PFR v...
Questions

English, 19.11.2019 18:31



English, 19.11.2019 18:31

Social Studies, 19.11.2019 18:31




Mathematics, 19.11.2019 19:31

Mathematics, 19.11.2019 19:31

Mathematics, 19.11.2019 19:31

Computers and Technology, 19.11.2019 19:31



Chemistry, 19.11.2019 19:31

History, 19.11.2019 19:31




English, 19.11.2019 19:31

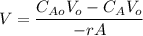
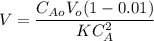
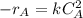 and k = 3 dm³/mol.h
and k = 3 dm³/mol.h
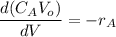
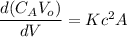
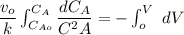
![V = \dfrac{V_o}{K} \Big [\dfrac{1}{C_A}- \dfrac{1}{C_{Ao}} \Big ]](/tpl/images/1098/6466/910c8.png)
![V= \dfrac{(10 \ dm^3/h) }{(3 \ dm^3/mol.h)}\Big [ \dfrac{1}{0.01(0.5 \ mol/dm^3)} - \dfrac{1}{0.5 \ mol/dm^3} \Big ]](/tpl/images/1098/6466/f41db.png)