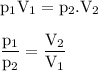Chemistry, 06.02.2021 06:00 tedrayoung1
The pressure of a sample of helium in a 200. ml. container is 2.0 atm. If the 5 points
helium is compressed toa volume of 10 ml without changing the
temperature, what would be the pressure of the gas? *
4000 atm
1000 atm
40 atm
0.1 atm

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Esign techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 3

Chemistry, 22.06.2019 02:30
Margaret wants to make an orange flavored drink by stirring powdered drink mix into a glass of water. she doesn't like drinks that have small clumps of powdered solid in them, so she wants the drink to be a perfect solution. what factors should margaret not consider when deciding how much powder to add to her glass of water?
Answers: 3

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3
You know the right answer?
The pressure of a sample of helium in a 200. ml. container is 2.0 atm. If the 5 points
helium is co...
Questions

History, 03.07.2019 09:20


Mathematics, 03.07.2019 09:20

Mathematics, 03.07.2019 09:20

Mathematics, 03.07.2019 09:20

Mathematics, 03.07.2019 09:20




English, 03.07.2019 09:20


Social Studies, 03.07.2019 09:20




Mathematics, 03.07.2019 09:20

Chemistry, 03.07.2019 09:20

Mathematics, 03.07.2019 09:20


Mathematics, 03.07.2019 09:20