

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2


Chemistry, 22.06.2019 07:00
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (ii) chloride. a single replacement reaction takes place. (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction? a) approximately 0.36 grams, because copper (ii) chloride acts as a limiting reactant b) approximately 1.8 grams, because copper (ii) chloride acts as a limiting reactant c) approximately 0.36 grams, because aluminum acts as a limiting reactant d) approximately 1.8 grams, because aluminum acts as a limiting reactant
Answers: 3

You know the right answer?
Some antacid tables contain aluminum hydroxide. The aluminum hydroxide reacts with stomach acid acco...
Questions

Mathematics, 16.02.2022 23:30






Physics, 16.02.2022 23:30


Biology, 16.02.2022 23:30

Mathematics, 16.02.2022 23:30


Social Studies, 16.02.2022 23:30

Mathematics, 16.02.2022 23:30




Mathematics, 16.02.2022 23:40


History, 16.02.2022 23:40

Mathematics, 16.02.2022 23:40

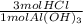 = 26.67 mol HCl
= 26.67 mol HCl

