
Chemistry, 07.02.2021 18:30 taniyahbenyamin2
A box has a volume of 45m3 and is filled with air held at 25∘C and 3.65atm. What will be the pressure (in atmospheres) if the same amount of air is placed in a box with a volume of 5.0m3 at 35∘C? Report your answer with two significant figures.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:20
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 09:30
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone,due to its high light availability and warm water temperature
Answers: 3

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

You know the right answer?
A box has a volume of 45m3 and is filled with air held at 25∘C and 3.65atm. What will be the pressur...
Questions

English, 04.07.2019 13:30

Mathematics, 04.07.2019 13:30




Physics, 04.07.2019 13:30




Social Studies, 04.07.2019 13:30

Mathematics, 04.07.2019 13:30



Physics, 04.07.2019 13:30





Biology, 04.07.2019 13:30

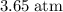 .Volume was reduced from
.Volume was reduced from 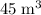 to
to 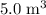 .Temperature was raised from
.Temperature was raised from 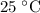 to
to 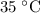 .
.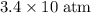 (
(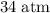 .) (Assuming that the gas is an ideal gas.)
.) (Assuming that the gas is an ideal gas.) 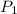 .Raise the temperature of the gas from
.Raise the temperature of the gas from 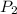 .
.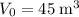 while
while 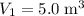 .
.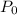 denote the pressure of this gas before the volume change (
denote the pressure of this gas before the volume change (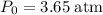 .) Let
.) Let 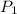 and
and 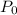 :
: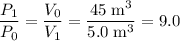 .
.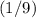 of the initial volume, the final pressure is
of the initial volume, the final pressure is 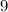 times the initial pressure. Therefore:
times the initial pressure. Therefore: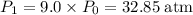 .
.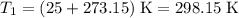 .
.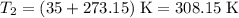 .
.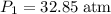 .) Let
.) Let 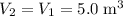 .
. 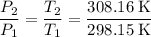 .
.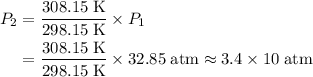 .
.

