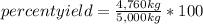A chemical process used to produce ethanol as a fuel additive was expected to produce 5,000 kilograms of ethanol based on the amounts of starting materials used, but only 4,760 kilograms were produced. What was the percent yield for ethanol in this process? A)1.09 percent B)4.80 percent C)95.2 percent D)105 percent

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3

Chemistry, 23.06.2019 01:30
The solubility of barium nitrate is 9.02 g/100 g h2o at 20°c. a 15.2 g sample of barium nitrate is added to 200.0 g of water at 20°c. is the solution saturated, unsaturated, or supersaturated? a. unsaturated b. saturated c. supersaturated
Answers: 1
You know the right answer?
A chemical process used to produce ethanol as a fuel additive was expected to produce 5,000 kilogram...
Questions



Mathematics, 03.12.2021 01:00


Physics, 03.12.2021 01:00

Advanced Placement (AP), 03.12.2021 01:00




Mathematics, 03.12.2021 01:00

World Languages, 03.12.2021 01:00

Chemistry, 03.12.2021 01:00

Mathematics, 03.12.2021 01:00





Advanced Placement (AP), 03.12.2021 01:00