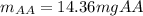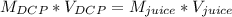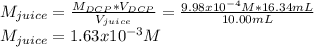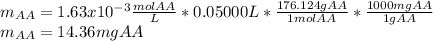Chemistry, 08.10.2019 18:30 annadel742
Students performed a procedure similar to part ii of this experiment (analyzing juices for vitamin c content) as described in the procedure section. three 10.00ml samples of juice were titrated with dcp that had a standardized concentration of 9.98x10-4m. the three titrations took an average of 16.34ml of dcp. calculate the mass (in mg) in 50.00ml of juice. (mm ascorbic acid = 176.124 g/mol)

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 19:00
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1

Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2

Chemistry, 22.06.2019 23:30
The density of benzene at 15 °c is 0.8787 g/ml. calculate the mass of 0.1500 l of benzene at this temperature. enter your answer in terms of grams
Answers: 2
You know the right answer?
Students performed a procedure similar to part ii of this experiment (analyzing juices for vitamin c...
Questions


Biology, 04.02.2020 02:54




English, 04.02.2020 02:54

Mathematics, 04.02.2020 02:54

History, 04.02.2020 02:54

Mathematics, 04.02.2020 02:54

Chemistry, 04.02.2020 02:54

Social Studies, 04.02.2020 02:54

Biology, 04.02.2020 02:54

Biology, 04.02.2020 02:54

Mathematics, 04.02.2020 02:54




Mathematics, 04.02.2020 02:54