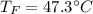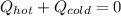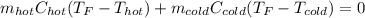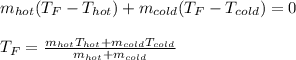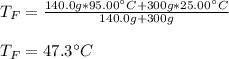Answers: 3


Another question on Chemistry



Chemistry, 23.06.2019 00:00
Mercury turns to a vapor at 629.88 k. how much heat is lost when 75.0 g of mercury vapor at 650 k condenses to a liquid at 297 k?
Answers: 1

You know the right answer?
If you combine 300.0 mL of water at 25.00 ∘C and 140.0 mL of water at 95.00 ∘C, what is the final te...
Questions

English, 29.01.2021 18:50

Mathematics, 29.01.2021 18:50


Mathematics, 29.01.2021 18:50





Social Studies, 29.01.2021 18:50


Mathematics, 29.01.2021 18:50



Social Studies, 29.01.2021 18:50

Mathematics, 29.01.2021 18:50

Advanced Placement (AP), 29.01.2021 18:50



Mathematics, 29.01.2021 18:50

Biology, 29.01.2021 18:50