Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which best describes how johannes kepler developed his laws of planetary motion
Answers: 3

Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2


Chemistry, 23.06.2019 02:00
The plant food contains nh4)3po4 what tests would you run to verify the presence of the nh4 ion and the po4 ion
Answers: 2
You know the right answer?
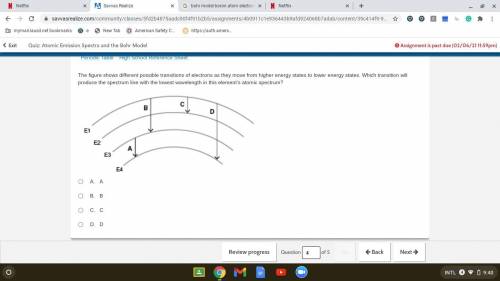
The figure shows different possible transitions of electrons as they move from higher energy states...
Questions




History, 24.07.2019 20:00


English, 24.07.2019 20:00


Advanced Placement (AP), 24.07.2019 20:00

Physics, 24.07.2019 20:00

Advanced Placement (AP), 24.07.2019 20:00

Mathematics, 24.07.2019 20:00

Geography, 24.07.2019 20:00

Physics, 24.07.2019 20:00



History, 24.07.2019 20:00

English, 24.07.2019 20:00



Spanish, 24.07.2019 20:00