
Chemistry, 08.02.2021 21:40 sillyvanna
PLEASE HELP ASAP WILL GIVE BRAINLIEST!!
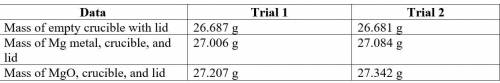
2. Subtract the mass of the crucible and lid (row 1 in the chart) from the total mass of Mg, crucible, and lid (row 2 in the chart) to find the mass of magnesium for each trial.
• Trial 1:
• Trial 2:
3. Subtract the mass of the crucible and lid (row 1 in the chart) from the total mass of MgO, crucible, and lid (row 3 in the chart) to find the mass of magnesium oxide for each trial. This is the actual yield of magnesium oxide for each trial.
• Trial 1:
• Trial 2:
4. Magnesium is the limiting reactant in this experiment. Calculate the theoretical yield of MgO for each trial.
• Trial 1:
• Trial 2:
5. Determine the percent yield of MgO for your experiment for each trial.
• Trial 1:
• Trial 2:
6. Determine the average percent yield of MgO for the two trials.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Check the correct box to describe the periodic trends in electronegativity. electronegativity across a period: decreases. increases. electronegativity down a group: decreases. increases.
Answers: 2

Chemistry, 21.06.2019 22:40
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration?
Answers: 3

Chemistry, 22.06.2019 00:00
What stress will shift the following equilibrium system to the left? n2(g) + 3h2(g) ⇌ 2nh3(g) adding more n2(g) adding more nh3(g) increasing the pressure of the system reducing the volume of the container
Answers: 1

You know the right answer?
PLEASE HELP ASAP WILL GIVE BRAINLIEST!!
2. Subtract the mass of the crucible and lid (row 1 in the...
Questions

English, 05.11.2020 22:00

Mathematics, 05.11.2020 22:00


Mathematics, 05.11.2020 22:00



Mathematics, 05.11.2020 22:00


History, 05.11.2020 22:00

Mathematics, 05.11.2020 22:00

History, 05.11.2020 22:00


Mathematics, 05.11.2020 22:00


Mathematics, 05.11.2020 22:00


Mathematics, 05.11.2020 22:00

Biology, 05.11.2020 22:00

Physics, 05.11.2020 22:00

Mathematics, 05.11.2020 22:00



