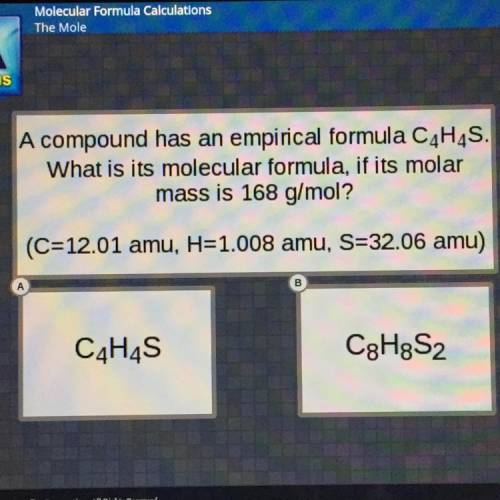
A compound has an empirical formula C4H4S.
What is its molecular formula, if its molar
mass i...

Chemistry, 09.02.2021 01:00 natalyarenassalgado
A compound has an empirical formula C4H4S.
What is its molecular formula, if its molar
mass is 168 g/mol?
(C=12.01 amu, H=1.008 amu, S=32.06 amu)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
If 90.0 grams of ethane reacted with excess chlorine,how many grams of dicarbon hexachloride would form
Answers: 1

Chemistry, 22.06.2019 09:50
What are four significant sources of ghgs that come from wostem washington?
Answers: 2

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 14:30
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2
You know the right answer?
Questions






Mathematics, 16.11.2020 20:40

Mathematics, 16.11.2020 20:40

Mathematics, 16.11.2020 20:40

Advanced Placement (AP), 16.11.2020 20:40

English, 16.11.2020 20:40


Mathematics, 16.11.2020 20:40





Arts, 16.11.2020 20:40



Mathematics, 16.11.2020 20:40



