Chemistry, 09.02.2021 01:00 thomasmurphy200
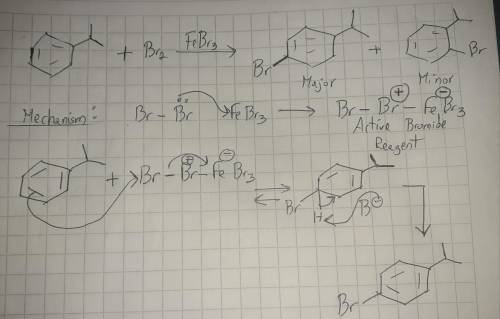
The electrophilic aromatic substitution of isopropylbenzene with FeBr3, Br2 gives 1-bromo-4-isopropylbenzene. Complete the curved-arrow mechanism below, beginning with formation of the active brominating reagent. Remember to include lone pairs and formal charges where appropriate.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:50
Your roll: experienced electron speech is adressed to: a new "freshman class" of electrons job: write a speech task: you are to pretend that you are giving a speech to a new group of electrons. be sure to mention their placement in an atom, their charge, and their role in chemical bonding (ionic and covalent) be specific!
Answers: 3


Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2

You know the right answer?
The electrophilic aromatic substitution of isopropylbenzene with FeBr3, Br2 gives 1-bromo-4-isopropy...
Questions

Mathematics, 23.12.2020 20:00



Chemistry, 23.12.2020 20:00




Mathematics, 23.12.2020 20:00

Mathematics, 23.12.2020 20:00

English, 23.12.2020 20:00


Mathematics, 23.12.2020 20:00

Mathematics, 23.12.2020 20:00



Mathematics, 23.12.2020 20:00




History, 23.12.2020 20:00