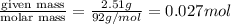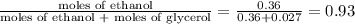Glycerol (C3H8O3), also called glycerine, is widely used in the food and pharmaceutical industries. Glycerol is polar and dissolves readily in water and polar organic solvents like ethanol. Calculate the mole fraction of the solvent in a solution that contains 2.51 g glycerol dissolved in 21.10 mL ethanol (CH3CH2OH; density

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Your friend offers to show you an intrusive igneous rock. which of the following would you expect to see?
Answers: 1

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2


You know the right answer?
Glycerol (C3H8O3), also called glycerine, is widely used in the food and pharmaceutical industries....
Questions



Mathematics, 03.02.2021 20:20


English, 03.02.2021 20:20

Mathematics, 03.02.2021 20:20

Biology, 03.02.2021 20:20

Mathematics, 03.02.2021 20:20





Mathematics, 03.02.2021 20:20

History, 03.02.2021 20:20

Computers and Technology, 03.02.2021 20:20

Mathematics, 03.02.2021 20:20


Mathematics, 03.02.2021 20:20

Mathematics, 03.02.2021 20:20

History, 03.02.2021 20:20