
Chemistry, 09.02.2021 01:00 eburnhisel2023
Given the following enthalpies of formation calculate the change in enthalpy for the reaction. Then justify if the reaction is endothermic or exothermic based on your calculations. PLEASE HELP WILL GIVE BRAINLIEST
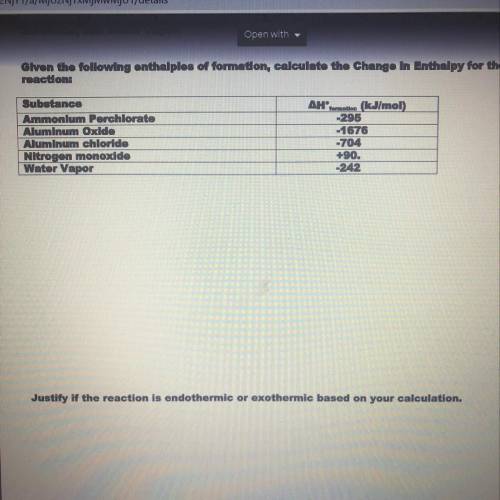

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Use the drop-down menus to answer each question. which runner finished the 100 m race in the least amount of time? which runner stopped running for a few seconds during the race? at what distance did anastasia overtake chloe in the race?
Answers: 1

Chemistry, 22.06.2019 21:20
The organs inside the body and how they function together
Answers: 3

Chemistry, 22.06.2019 22:00
If a solution contains 3 moles/liter of sodium chloride (nacl, made of sodium ions and chloride ions), what is the osmolarity of this solution
Answers: 3

Chemistry, 23.06.2019 02:20
Which of the following will cause an increase in the acceleration of an object? increase force decrease force increase mass decrease mass
Answers: 1
You know the right answer?
Given the following enthalpies of formation calculate the change in enthalpy for the reaction. Then...
Questions

Mathematics, 19.06.2020 17:57



Mathematics, 19.06.2020 17:57














Chemistry, 19.06.2020 17:57

Mathematics, 19.06.2020 17:57

Biology, 19.06.2020 17:57



