
Chemistry, 09.02.2021 01:50 courtney3652
The equilibrium constant, Kc, for the following reaction is 83.3 at 500 K. PCl3(g) Cl2(g) PCl5(g) Calculate the equilibrium concentrations of reactant and products when 0.453 moles of PCl3 and 0.453 moles of Cl2 are introduced into a 1.00 L vessel at 500 K.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1

Chemistry, 22.06.2019 22:00
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3
You know the right answer?
The equilibrium constant, Kc, for the following reaction is 83.3 at 500 K. PCl3(g) Cl2(g) PCl5(g) Ca...
Questions

English, 23.02.2021 21:00

Mathematics, 23.02.2021 21:00


Social Studies, 23.02.2021 21:00

Mathematics, 23.02.2021 21:00

English, 23.02.2021 21:00

Arts, 23.02.2021 21:00




History, 23.02.2021 21:00

History, 23.02.2021 21:00

Mathematics, 23.02.2021 21:00

English, 23.02.2021 21:00

Mathematics, 23.02.2021 21:00

Mathematics, 23.02.2021 21:00

Mathematics, 23.02.2021 21:00

Mathematics, 23.02.2021 21:00

History, 23.02.2021 21:00

![[PCl_3]=[Cl_2]=0.068M](/tpl/images/1103/3296/1ad55.png)
![[PCl_5]=0.385M](/tpl/images/1103/3296/9d9e6.png)
![Kc=\frac{[PCl_5]}{[Cl_2][PCl_3]}](/tpl/images/1103/3296/6b2bc.png)
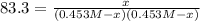
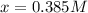
![[PCl_3]=[Cl_2]=0.453M-0.385M=0.068M](/tpl/images/1103/3296/82c25.png)



