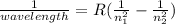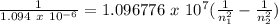Answers: 3


Another question on Chemistry

Chemistry, 20.06.2019 18:04
What is the molarity of a solution that is prepared by adding 57.1 g of toluene (c 7 h 8 ) (density = 0.867 g/ml) to a 250 ml volumetric flask, and then filling to the mark with benzene (c 6 h 6 ) (density = 0.876 g/ml)?
Answers: 1

Chemistry, 21.06.2019 22:30
Explain why scientists use shared characteristics to make cladograms.
Answers: 1

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1

You know the right answer?
The hydrogen atom can absorb light of wavelength 1094 nm. find the initial and final values of n ass...
Questions

History, 23.03.2021 19:30

Mathematics, 23.03.2021 19:30

Mathematics, 23.03.2021 19:30


Mathematics, 23.03.2021 19:30






Mathematics, 23.03.2021 19:30





English, 23.03.2021 19:30


Mathematics, 23.03.2021 19:30


Chemistry, 23.03.2021 19:30